What are the viable alternatives to chrome coatings?
20 June 2023While they had been known since the 1950’s, the potential dangers of chromium were brought to the public’s attention in the 1999 film Erin Brockovich. There, the title character fought to expose the toxicity of the hexavalent chromium that had seeped into a local water supply from a power plant. A chromium coating had been used as a rust inhibitor, preventing piston engines from corroding when water was used to cool them down. The film was of course based on the real life of Erin Brockovich: thanks in part to her pioneering work, the dangers of industrial chromium have become lodged in the public mind.
What's the harm?
Of all forms of chromium compounds, hexavalent chromium (chromium(VI)), has the most extreme effect on health. Its high solubility in water means it can enter the body through skin contact, inhalation, or swallowing food that has come into contact with chromium. The toxicity of other chromium forms has not been as widely studied, although the low solubility of chromium metal and chromium(III) mean that they are both considered to be safe.
Repeated exposure to hexavalent chromium has been found to cause cancer, organ damage, and issues in human reproduction. This affects both those working in close proximity to chromium, and communities where industry leads to a build-up of the chemical in the air, ground, and water. Steel workers working on alloys containing chromium, workers at cement plants, and workers using paints containing anti-corrosion chromium all face the high long-term risks of hexavalent chromium’s industrial use.
Changing patterns of chromium use
For decades chromium had been extensively used in many industries to provide wear- and corrosion-resistance. Today, the use of chromium is increasingly widely monitored and regulated. While chromium is not banned from all industrial uses, the need for employers to ensure the health and safety of their workers and the environment means that chromium use must be kept to an absolute minimum. As a result, finding alternatives has become imperative and there is an increasing range of viable options with reduced safety concerns.
Chrome plating
Chrome plating once proved a highly effective way to protect metals from corrosion. Chrome plated car and lorry parts remain common in the automotive industry. A chromium coating is applied to a base metal via electroplating, for decorative purposes and to protect against corrosion. Hexavalent chromium trioxide and sulphuric acid are typically used in the electrolyte during the plating process. As a result, while the resultant coating is non-toxic, the electrolyte and waste products of the process are highly toxic, posing a health risk to workers and the local environment.
New coating technology such as plasma electrolytic oxidation (PEO) forms an extremely strong, corrosion resistant surface layer from a substrate of lightweight metal such as aluminium, magnesium or titanium. The PEO process involves passing a higher voltage through the metal than is typically used during anodisation to create discharges that result in plasma, forming a highly resistant oxide on the metal’s surface. Typical coatings produced by PEO have no toxic byproducts, making it a green procedure with all the wear resistance of chrome plating.
Paints and surface coatings
Chromium(VI) compounds were commonly used as anticorrosive agents in paints, inks, and other surface coatings. There are numerous other surface coating solutions, such as paints, primers and other polymeric systems, with none of the toxicity hazards and a wide variety of properties and aesthetic qualities. Powder coatings may also be considered. For larger scales, PEO may provide a lighter, more economical replacement to surface coatings, where the tendency for coatings to evaporate during application leads to inefficiency and waste.
Chromate conversion processes
One potential advantage of chromium is its low electrical resistance, making it a usefully resilient material for grounding components and creating Faraday cages. It had been thought that the coating formed during PEO had to be an electrical insulator, perhaps preventing its use as a green alternative to chromium. However, a major benefit of PEO is the high degree of accuracy with which the variables involved in the coating process can be controlled. Through careful attention to variables, Keronite have explored a method known as ‘Type C’ coating. Their research into the Type C coating has shown that PEO can be controlled to produce an extremely resistant coating on aerospace-grade magnesium alloys (Elektron 21 and WE43) that also has low electrical resistance. Research conducted by a major car company found that the PEO surface treatment Keronite is “the only system to exceed the protection offered by chromium(VI) conversion”.
This image demonstrates the relative levels of corrosion of several types of conductive coating, include Keronite Type C and chromium(VI) based coatings, after various periods of exposure to corrosion:
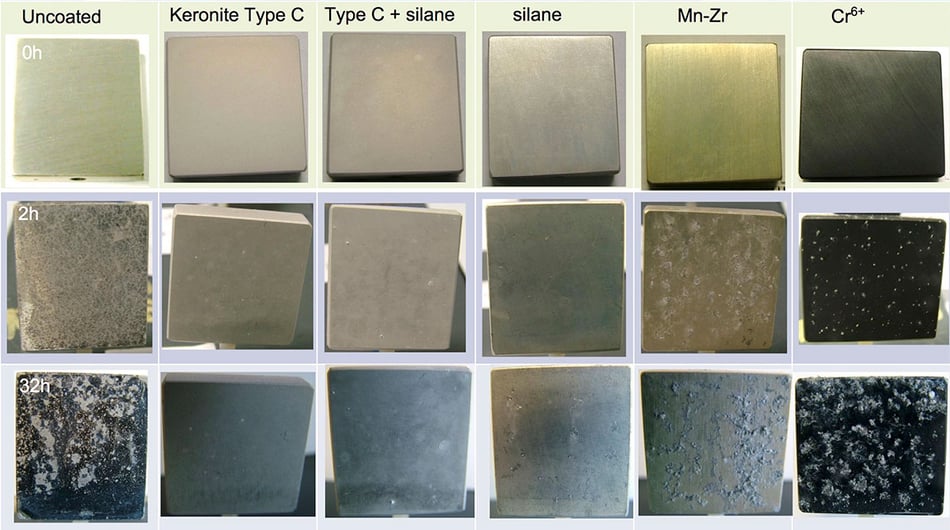
As can be seen, Type C PEO coating shows superior corrosion protection whilst retaining electrical continuity after 32 hours of exposure to salt fog.
Increased pressure to avoid the toxic and environmental hazards that come with chromium coatings have led industry to look elsewhere for strong, light corrosion resistance. This change of focus has revealed the potential of technology like PEO, at last offering viable green alternatives to chromium.
Keronite “Type C” PEO coating has been developed - for corrosion resistance with low electrical resistance - and tested on E21 and WE43 alloys alongside other commercial treatments. For more information about Keronite coatings, feel free to ask us a question by getting in touch.

 Keronite is now part of the CWST engineered coatings business.
Keronite is now part of the CWST engineered coatings business.